Critical care, a forefront arm of Pharmika plays an eminent role in the manufacturing of recently approved finished formulations of critical care products. The need for more advanced critical care has inspired pharmika to foster the technology and put the expertise of its researchers at work in the development of critical care products. We aim at including all the aspects of the disease and the medicines in development of critical care products that works on varied ailments – antibiotics, antifungals, antivirals, anti-infectives etc.
The production facility at Pharmika is developed taking into account various safety and quality standards. We adhere to various safety measures in order to safeguard the working environment and interests of our personnel. The ultra-modern production units at Pharmika include dedicated areas for research, production and packaging. These units are designed to match international standards.
The varied range of critical care products manufactured by Pharmika helps recover patients and to prevent and reduce the risks induced by high resistant infections for patients in critical care units, pre & post surgical complications & infectious diseases. The critical care production unit is into manufacturing of various injectable, dry powder inhaler, Lyophilized injections, tablets and prefilled syringes. Our team has been successful in developing next generation Carbapenems like Ertapenem, Doripenem. Our manufacturing includes some of the commonly used ICU products like Amoxcillin/Clavulanic acid, Azithromycin, Heparin, Paracetamol injections, Vancomycin etc.
| S. No | Product Name | Dosage Form | Strength |
| 1 | Azithromycin for injection | Lyophilizate | 0.5 g |
| 2 | Clarithromycin for injection | Lyophilizate | 0.5 g |
| 3 | Tigecycline for injection | Lyophilizate | 50 mg |
| 4 | Teicoplanin for injection | Lyophilizate | 0.2 g & 0.4 g |
| 5 | Polymyxin B for injection | Lyophilizate | 25 mg & 50 mg |
| 6 | Vancomycin + WFI | Dry powder injection | 0.5 g & 1 g |
| 7 | Meropenem for injection | Dry powder injection | 0.5 g & 1 g |
| 8 | Imipenem & Cilastatin for injection | Dry Powder injection | 0.5 g |
| 9 | Doripenem for injection | Dry Powder injection | 0.5 g |
| 10 | Ertapenem for injection | Lyophilizate | 1 g |
| 11 | Enoxaparin PFS | PFS | 20,40,60,80 & 100 mg |
| 12 | Heparin PFS | Liquid injection | 5000 IU |
| 13 | Omeprazole for injection | Lyophilizate | 20 mg |
| 14 | Levosimendan | IV infusion | 12.5 mg & 25 mg |
| 15 | Eptifibatide | Liquid injection | 20 mg & 75 mg |
| 16 | Bivalirudin | Lyo injection | 250 mg |
| 17 | Colistemethate Sodim for injection | Lyophilizate | 1 M & 2 M IU |
| 18 | Caspofungin for injection | Lyophilizate | 50 mg & 70 mg |
| 19 | Voriconazole for injection | Lyophilizate | 0.2 g |
| 20 | Daptomycin for injection | Lyophilizate | 0.35 g & 0.5 g |
| S. No | Product Name | Product information |
| 1 | Clarithromycin | |
| 2 | Meropenem | |
| 3 | Piperacillin + Tazobactam | |
| 4 | Enoxaparin |
Cephalosporins, a new class of antibiotics are an incredible part of the medicine world. Cephalosporins are developed to fight against bacteria and used as first line antibiotics to inhibit bacterial growth in human body. It is often used as an alternative for penicillin in fighting infections.
The line of production set for cephalosporin needs high level of precision and control. Pharmika holds a capacity to produce both oral and injectable cephalosporins. Our production capacity is designed, taking into account the globally accepted medical norms and regulations. We at Pharmika put in expert intelligence and modern technology in controlling the manufacturing of cephalosporins. We deliver consistent high quality and reliable products.
Cephalosporins unit have capacity to manufacture 25 million vials of sterile dry powder for injection per annum, 230 mln oral dosage of tablets and 160 mln oral dosage of capsules per annum.
Cephalosporin’s Product Information
| S. No | Product Name | Dosage Form | Strength |
| 1 | Ceftriaxone for injection USP + WFI | Dry Powder injection | 0.5 g & 1 g |
| 2 | Cefepime for injection USP + WFI | Dry Powder injection | 0.5 g & 1 g |
| 3 | Cefuroxime for injection USP + WFI | Dry Powder injection | 0.75 g & 1.5 g |
| 4 | Cephalothin for injection USP | Dry Powder injection | 1 g |
| 5 | Cefotaxime for injection | Dry Powder injection | 0.5 g & 1 g |
| 6 | Cefoperazone + Sulbactam for injection | Dry Powder injection | 1g + 1g |
| 7 | Ceftazidime for injection USP | Dry Powder injection | 0.5 g & 1 g |
| 8 | Cefoxitin for injection | Dry Powder injection | 2 g |
| 9 | Cefazolin for Injection | Dry Powder injection | 0.5 g & 1 g |
| 10 | Cefpirome for Injection | Dry Powder injection | 1 g |
| 11 | Ceftriaxone + Tazobactum injection | Dry Powder injection | 1 g + 0.125 g |
| 12 | Cefepime + Sulbactum Injection | Dry Powder injection | 0.5 g + 0.125 g |
| 13 | Ceftazidime + Avibactam injection | Dry Powder injection | 2 g + 0.5 g |
| 14 | Cefamandole nafate | Dry Powder injection | 1gm |

PlasmaHep is a sterile, clear to opalescent, colourless to pale yellow solution. It contains not more than 180g/L human plasma proteins of which at least 90% is human immunoglobulins (mainly gamma globulins IgG), with Hepatitis B antibody titer of not less than 200IU/mL. PlasmaHep contains more than 90% of monomeric and dimeric IgG.
To increase margin of safety, effective steps in extensive testing protocols have been integrated into manufacturing and formulation process to ensure contamination free product from HBsAg, anti- HIV I & II, anti-HCV, HBV-DNA, HCV-RNA, HIV-RNA by PCR.
30 months from the date of manufacture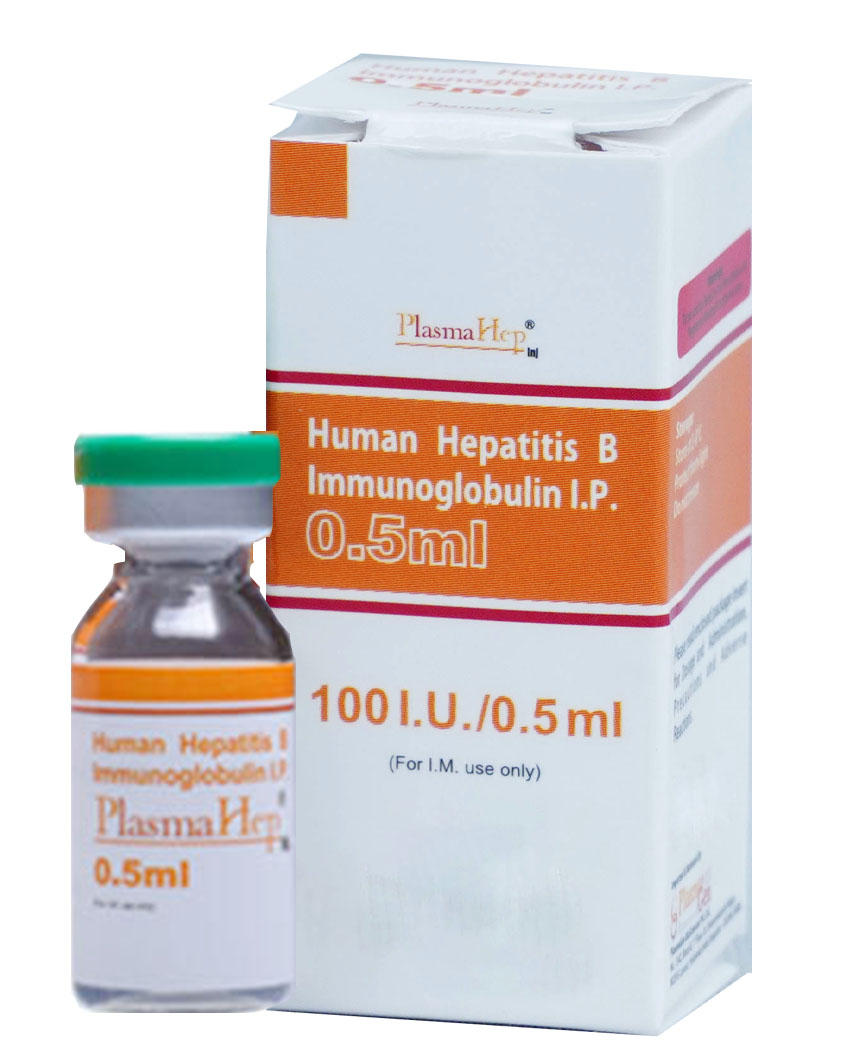
PlasmaHep
Human Hepatitis-B Immune Globulin
100 I.U. in 0.5 mL

PlasmaGlob is Normal Human Immunoglobulin for Intravenous use prepared from Indian Human Plasma from healthy voluntary donors, by Cohn Fractionation/ Chromatography process. Each unit of plasma is tested for all mandatory serological markers prescribed by National Regulatory Authority (NRA).
To increase safety profile of PlasmaGlob , the plasma used in its manufacturing and final product undergoes extensive testing from various protocols HBsAg, Ab-Ag HIV, anti-HCV by ELISA and for HBV-DNA, HCV-DNA, HAV-RNA, HIV-RNA, Parvovirus B19-DNA by NAT/PCR.
Only the non-reactive plasma units were used for manufacturing of PlasmaGlob. To further improve the margin of safety effective viral inactivation steps like solvent detergent treatment have been integrated into the manufacturing and formulation process. PlasmaGlob has undergone two steps of viral inactivation. As per the regulatory norms and Hemovigilance program, the record of plasma used in manufacturing of PlasmaGlob has been maintained for 10 years.
36 months from the date of manufacture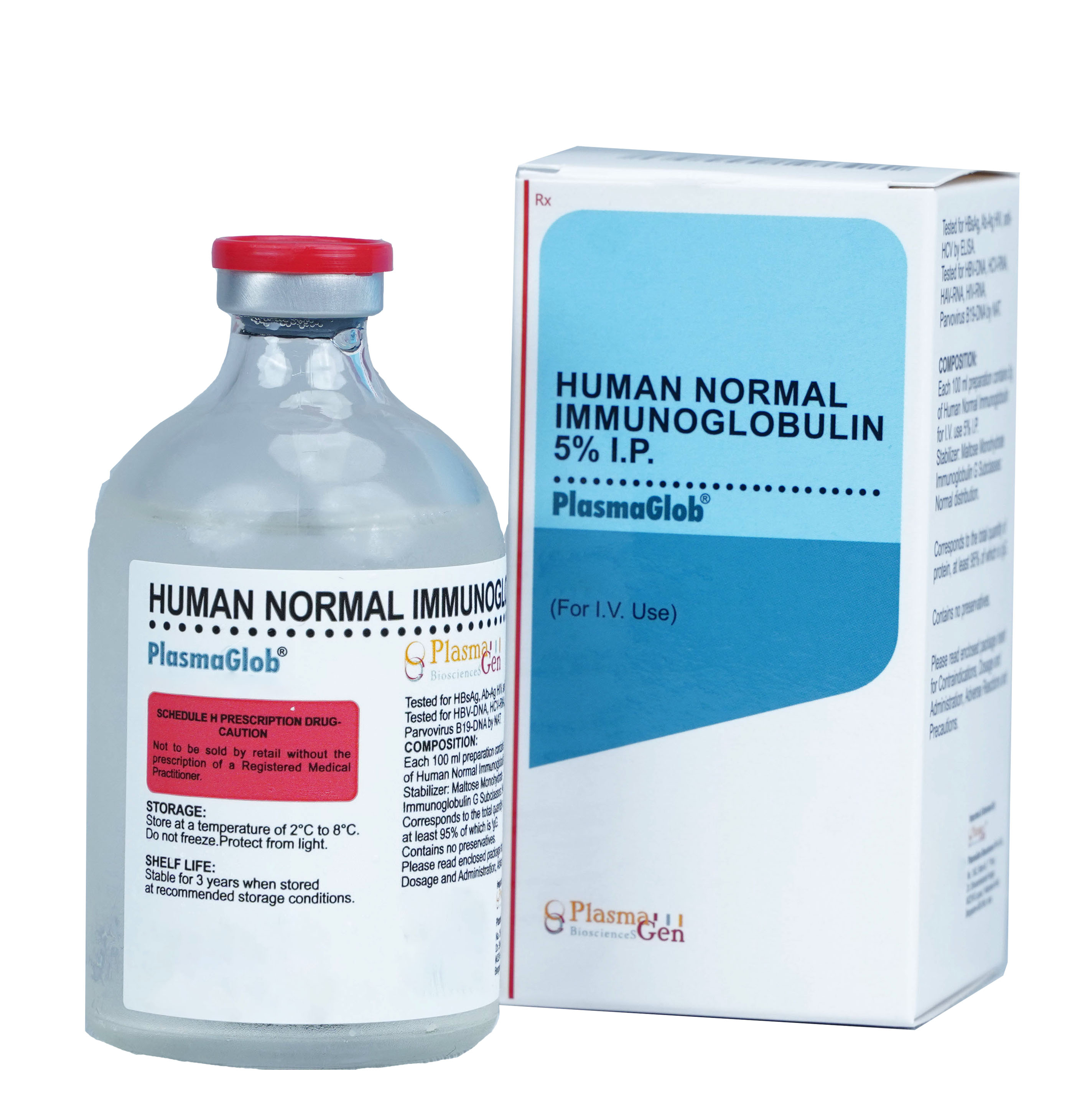
PlasmaGlob
Human Intravenous Immunoglobulin 5%
5% in 10 mL, 50mL, 100 mL [0.5g, 2.5g, 5g]
PlasmaGlob 10 is Normal Human Immunoglobulin, 10% in 50 mL & 100mL for Intravenous use prepared from Indian Human Plasma from healthy voluntary donors, by Cohn Fractionation/ Chromatography process. Each unit of plasma is tested for all mandatory serological markers prescribed by National Regulatory Authority (NRA).
To increase safety profile of PlasmaGlob 10, the plasma used in its manufacturing and final product undergoes extensive testing from various protocols HBsAg, Ab-Ag HIV, anti-HCV by ELISA and for HBV-DNA, HCV-DNA, HAV-RNA, HIV-RNA, Parvovirus B19-DNA by NAT/PCR.
Only the non-reactive plasma units were used for manufacturing of PlasmaGlob. To further improve the margin of safety effective viral inactivation steps like solvent detergent treatment have been integrated into the manufacturing and formulation process. PlasmaGlob has undergone two steps of viral inactivation. As per the regulatory norms and Hemovigilance program, the record of plasma used in manufacturing of PlasmaGlob has been maintained for 10 years.
PlasmaGlob 10 is Glycine stabilized IVIG, tested, developed, and documented in EU associates and other international markets and contains no preservatives.
36 months from the date of manufacture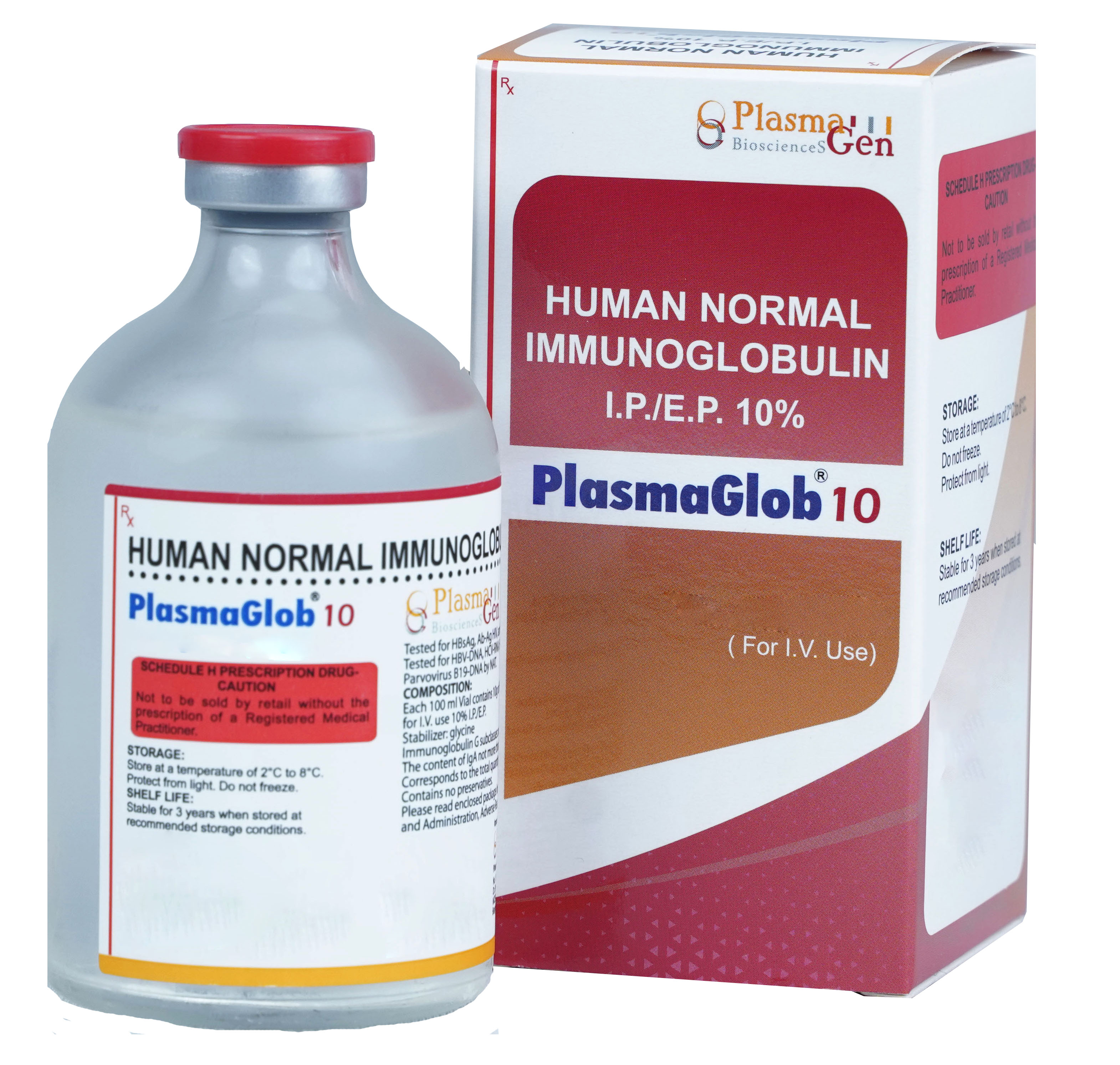
PlasmaGlob 10
Human Intravenous Immunoglobulin 10%
10% in 50 mL, 100 mL [5g, 10g]

PlasmaRAB is a sterile, non-pyrogenic aqueous solution containing not less than 150 I.U./mL of anti-rabies immune globulin (human). PlasmaRAB is supplied in 2mL single dose vial.
The product is assayed with human rabies immunoglobulin reference standard that is calibrated against the WHO International Standard. The product contains at least 95% immunoglobulin G (IgG).
30 months from the date of manufacture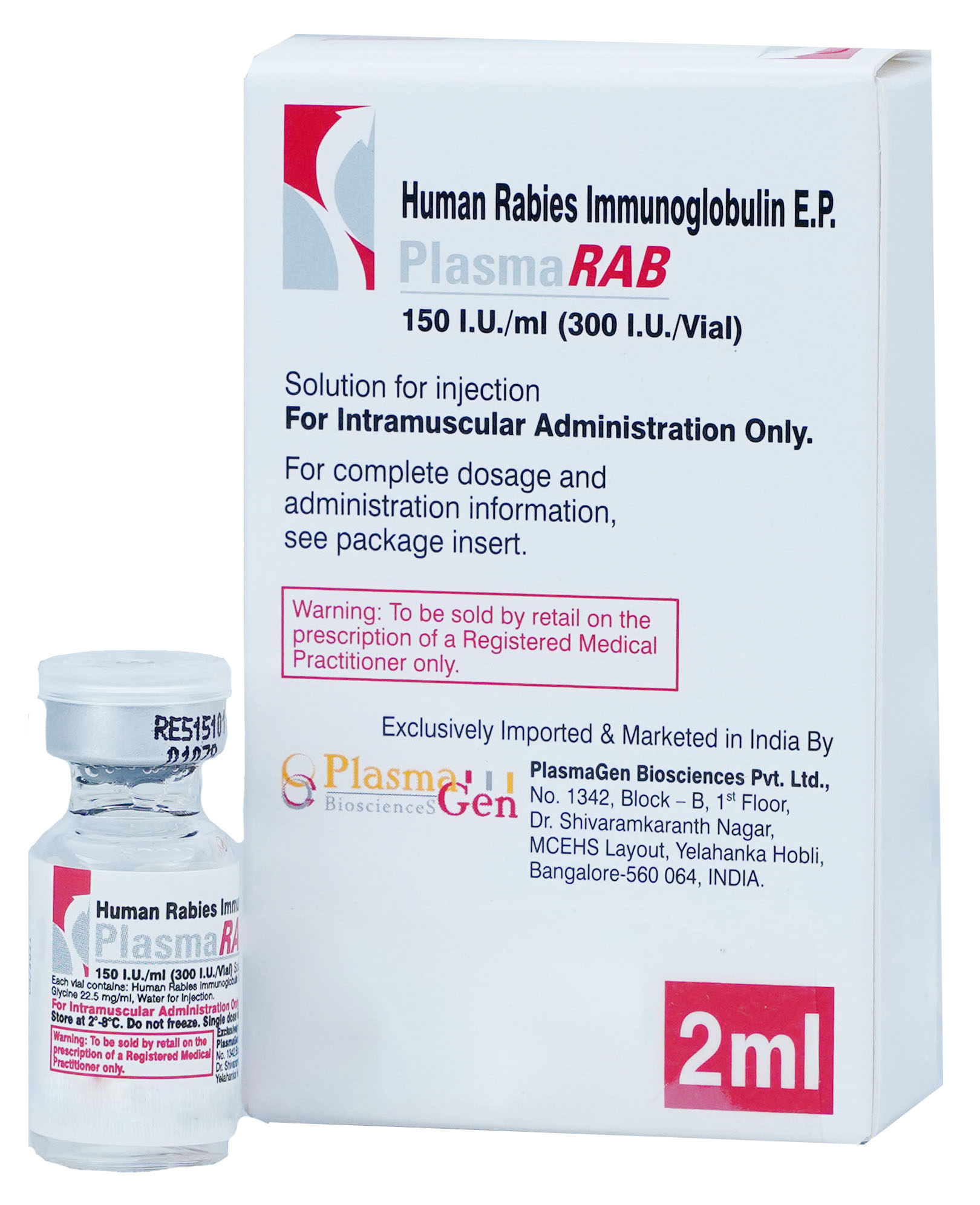
PlasmaRAB
Human Rabies Immune Globulin
300 I.U. in 2 mL [150 I.U./mL]

PlasmaRho-D I.V. is a sterile non-pyrogenic aqueous solution, containing 150 μg /mL (1500 I.U. in 2mL) of immune globulin Anti-D. PlasmaRho-D I.V. is prepared from pooled Human venous plasma with a high content of Anti-D antibodies by ion-exchange column chromatography.
Each unit of plasma and each plasma pool used in the manufacture of this product has been tested and found to be negative / non-reactive to Hepatitis B surface Antigen (HBsAg), anti-HIV I-II and anti-HCV. Additionally, the plasma is tested by NAT method for HAV, HIV, HBV, and HCV and to contain <104 I.U. / mL Parvovirus B-19. Also, the manufacturing process includes a Solvent/Detergent step and Heat-treatment for inactivation of non-enveloped viruses.
36 months from the date of manufacture
PlasmaRho-D I.V.
Human Anti-D Immune Globulin
300 μg in 2 mL [150 μg /mL]

AlbuMax(Normal Human Albumin 20%) is a sterile highly purified solution of Albumin protein prepared from Human Plasma collected from healthy voluntary donors. AlbuMax contains no preservatives.
To increase safety profile of AlbuMax, the plasma used in its manufacturing and final product undergoes extensive testing of various protocols; HBsAg, Ab-Ag HIV, anti-HCV by ELISA and for HBV-DNA, HCV-DNA, HAV-RNA, HIV-RNA, Parvovirus B19-DNA by NAT. To further improve the margin of safety, effective viral inactivation/removal steps have been integrated into the manufacturing and formulation process.
36 months from the date of manufacture
AlbuMax
Human Albumin Solution
20% in 100 mL

VerBumin is Normal Human Albumin for Intravenous use manufactured from Indian Human Plasma. Each unit of plasma is tested for all mandatory serological markers prescribed by National Regulatory Authority (NRA).
VerBumin undergoes heat pasteurization at 60°C for 10-12 hrs. for viral inactivation.
To increase margin of safety, effective steps in extensive testing protocols have been integrated into manufacturing and formulation process to ensure contamination free product from HBsAg, HCV, HIV antibodies and for HBV-DNA, HCV-RNA, HIV-RNA by PCR. As per regulatory norms and Hemovigilance program, the record of plasma used in manufacturing of Verbumin has been maintained for 10 years.
24 months from the date of manufacture
VerBumin
Human Normal Albumin
5% in 100 mL; 20% in 100 mL

AsparPeg (PEG Asparaginase) is L-asparaginase (L-Amidohydrolase) that is covalently conjugated to monomethoxypolyethelene glycol (mPEG). L- Asparaginase is a tetrameric enzyme that is produced endogenously by E. coli and consists of identical 34.5 kDa subunits. Approximately 69 to 82 molecules of mPEG are linked to L-Asparaginase; the molecular weight of each mPEG molecule is about 5kDa. The mechanism of action of PEG Asparaginase is thought to be based on selective killing of leukemic cells due to depletion of plasma asparagine.
24 months from the date of manufacture
AsparPeg
Peg L-Asparaginase
3750 I.U. in 5mL [750 I.U. / mL]

Avangio (Bevacizumab) is a recombinant humanized monoclonal IgG1 antibody that binds to and inhibits the biologic activity of human vascular endothelial growth factor (VEGF).
Avangio contains human framework regions and the complementarity-determining regions of a murine antibody that binds to VEGF. Avangio is produced in a Chinese Hamster Ovary mammalian cell expression system in a nutrient medium and has a molecular weight of approximately 149kDa.
24 months from the date of manufacture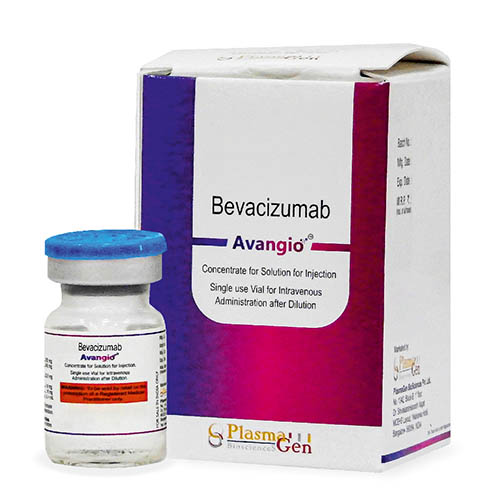
Avangio
Bevacizumab
100 mg in 4mL, 400 mg in 16mL

Ritulasta (Rituximab) is a genetically engineered chimeric murine/human monoclonal antibody directed against the CD20 antigen found on the surface of normal and malignant B lymphocytes. The antibody is an IgG1 kappa immunoglobulin containing murine light and heavy-chain variable region sequences and human constant region sequences. Rituximab is composed of two heavy chains of 451 amino acids and two light chains of 213 amino acids.
30 months from the date of manufacture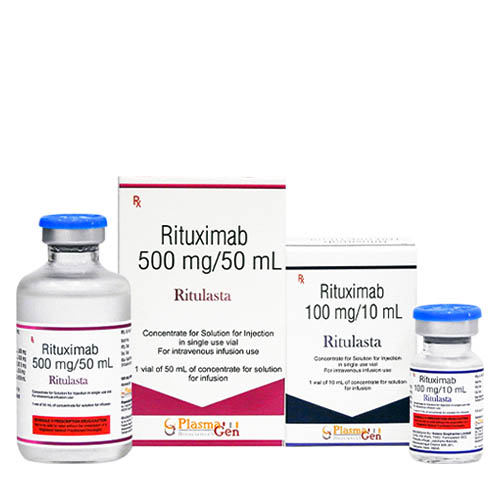
Ritulasta
Rituximab
100 mg in 10 mL, 500 mg in 50 mL

Mobixafor (Plerixafor) is a sterile, preservative-free, clear, colorless to pale yellow, isotonic solution for subcutaneous injection. Each mL of the sterile solution contains 20 mg of plerixafor. Each single-use vial is filled to deliver 1.2 mL of the sterile solution that contains 24 mg of plerixafor and 5.9 mg of sodium chloride in Water for Injection adjusted to á pH of 6.0 to 7.5 with hydrochloric acid and with sodium hydroxide, if required. Plerixafor is a hematopoietic stem cell mobilizer.
Mobixafor (Plerixafor) is indicated in combination with granulocyte-colony stimulating factor (G-CSF) to enhance mobilization of haematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation in adult patients with lymphoma or multiple myeloma whose cells mobilise poorly.
36 months from the date of manufacture
After opening-
From a microbiological point of view the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user.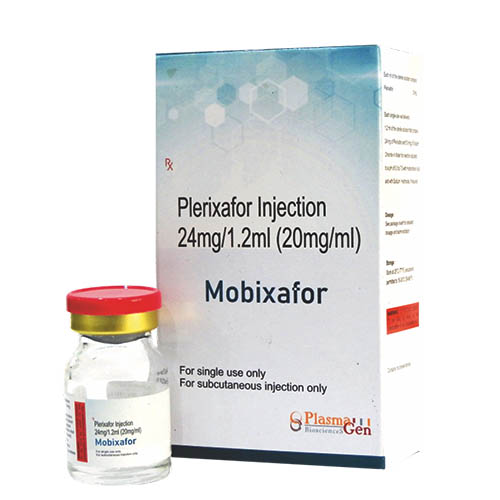
Mobixafor
Plerixafor
24 mg in 1.2mL [20mg / mL]

Ulipta (Ulinastatin) ) is a serine protease inhibitor that reduces the pro-inflammatory response as a result of sepsis, acute pancreatitis, trauma, or surgery. Ulinastatin for Injection is available as lyophilized powder for reconstitution.
24 months from the date of manufacture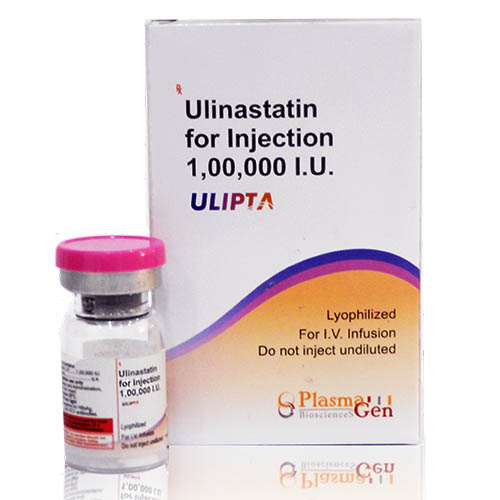
Ulipta
Ulinastatin
100,000 I.U. in 5 mL [20,000 I.U./mL]

Nabexol (Paclitaxel protein-bound particles for injectable suspension) is an albumin-bound form of Paclitaxel with a mean particle size of approximately 130 nanometers. The active agent in Nabexol is paclitaxel, a natural product with antitumor activity. Paclitaxel exists in the particles in a non-crystalline, amorphous state.
Nabexol is supplied as a white to yellow, sterile lyophilized powder for reconstitution with 20mL of 0.9% Sodium Chloride injection prior to intravenous infusion. Each single-use vial contains 100 mg of paclitaxel and approximately 900 mg of human albumin. Each milliliter (mL) of reconstituted suspension contains 5 mg paclitaxel. Nabexol is free of solvents.
24 months from the date of manufacture
Nabexol
Paclitaxel (protein-bound particles) for Injectable Suspension
100 mg of paclitaxel in a 50 mL

Sorakine (Sorafenib), is a multikinase inhibitor targeting several serine/threonine and receptor tyrosine kinases, is the tosylate salt of sorafenib. Sorafenib tosylate is a white to yellowish or brownish solid, with a molecular weight of 637.0 g/mole. Sorafenib tosylate is practically insoluble in aqueous media, slightly soluble in ethanol and soluble in PEG 400.
24 months from the date of manufacture
Sorakine
Sorafenib Tablets
200mg [Bottle of 30 and 60 Tablets]

DaPhila (Dasatinib) is a kinase inhibitor. The anhydrous free base has a molecular weight of 488.01. DaPhila tablets are white to off-white, biconvex, film-coated tablets containing Dasatinib. The drug substance is insoluble in water and slightly soluble in ethanol and methanol.
Tablets should not be crushed or cut; they should be swallowed whole. DaPhila inhibits the growth of chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL) cell lines overexpressing BCR-ABL.
Please see manufacturing date and expiry date printed on pack. Do not use the product after the expiry date which is stated on the package. The expiry date refers to the last day of the month.
Daphila
Dasatinib Tablets
20 mg & 50 mg [Bottle of 60 Tablets]

AlbuMax-Neo(Normal Human Albumin 20%) is a sterile highly purified solution of Albumin protein prepared from Human Plasma collected from healthy voluntary donors. AlbuMax-Neo contains no preservatives.
To increase safety profile of AlbuMax-Neo, the plasma used in its manufacturing and final product undergoes extensive testing of various protocols; HBsAg, Ab-Ag HIV, anti-HCV by ELISA and for HBV-DNA, HCV-DNA, HAV-RNA, HIV-RNA, Parvovirus B19-DNA by NAT. To further improve the margin of safety, effective viral inactivation/removal steps have been integrated into the manufacturing and formulation process.
36 months from the date of manufacture
AlbuMax-Neo
Human Albumin Solution
20% in 100 mL